Progressis

PRRS: still a big concern for the swine industry
PRRS still is a huge economic concern
- Economic losses vary from € 59 to € 379 per sow during an outbreak1
- Yearly cost of infected farms is around € 100 per sow1
PRRS is diffi cult to control:
- Emerging circulation of variable fi eld strains worldwide
- PRRS impairs immunity after infection:
- Immune response is slow, delayed and often incomplete in comparison with other viral infections
- Modified immune response after infection: increased susceptibility for other diseases
- Immunity is aff ected by strain diff erences
- Impaired reproductive and respiratory performances

PROGRESSIS®: boosting immunity in a PRRS positive environment
PROGRESSIS®: safety profile
- No reverse to virulence possible (inactivated virus)
- Approved for use during pregnancy and lactation2
- Neither spread nor persistence of vaccine virus
PROGRESSIS®: efficacy by boosting immunity
- Reduction of reproductive disorders caused by PRRS in a contaminated environment:
- Reduction of early farrowings (Fig. 1)
- Reduction of stillbirths (Fig. 1)
- Reduction of the number of viraemic piglets at weaning5 (Fig. 2)

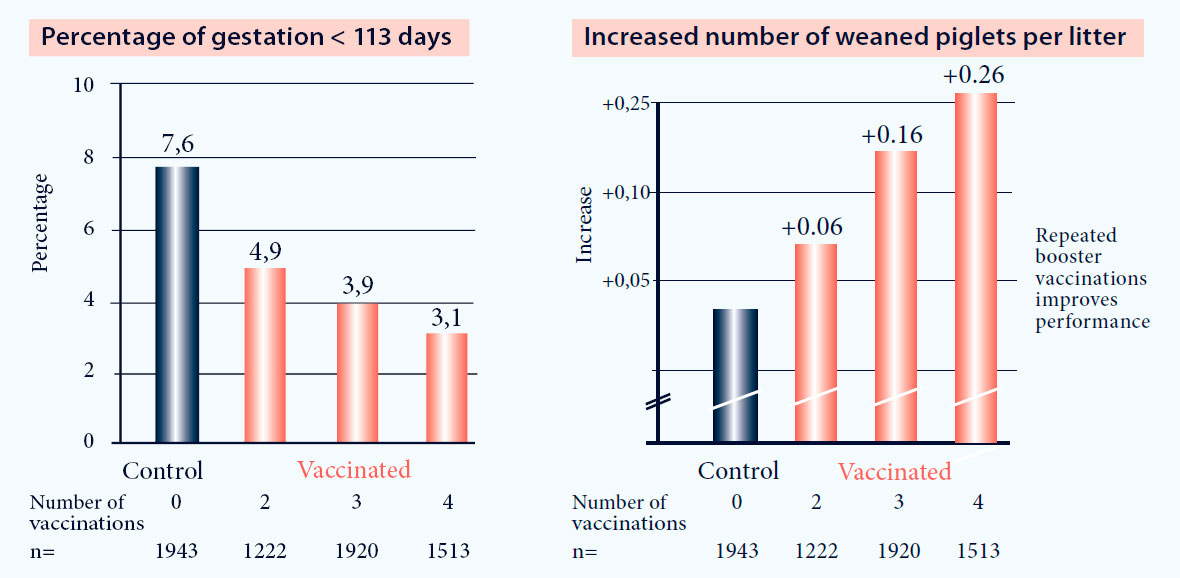
Fig. 1. Vaccination with Progressis in PRRS positive farms increases reproductive performance (p<0.05)3.
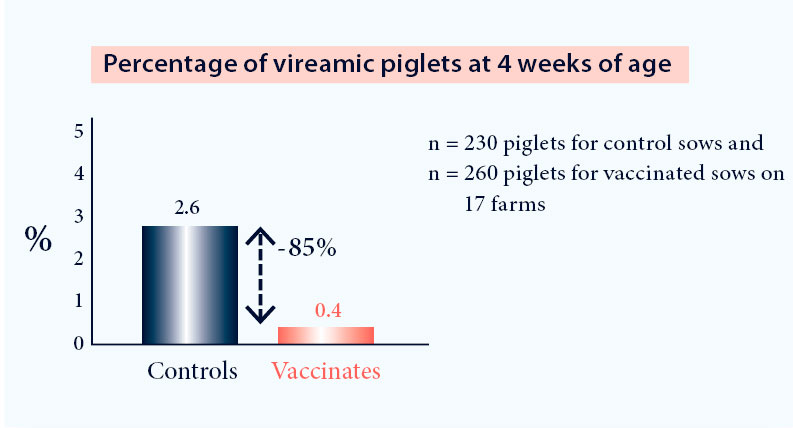
Fig. 2. Vaccination with Progressis in a PRRS positive farm reduces the number of PRRS viraemic piglets at weaning (p<0.05)4.
PRRS: immunity
Immunity against PRRS is based on both cell mediated and humoral immunity5
- Induction of immunity is slow
Both cell mediated and humoral immunity play a role in protection
- First (non-neutralizing) antibodies are present 1 to 2 weeks after infection
- Neutralizing antibodies are induced after 21 to 28 days
- Some strains do not induce signifi cant levels of neutralizing antibodies
- Cell mediated immunity (CMI) is slowly developing
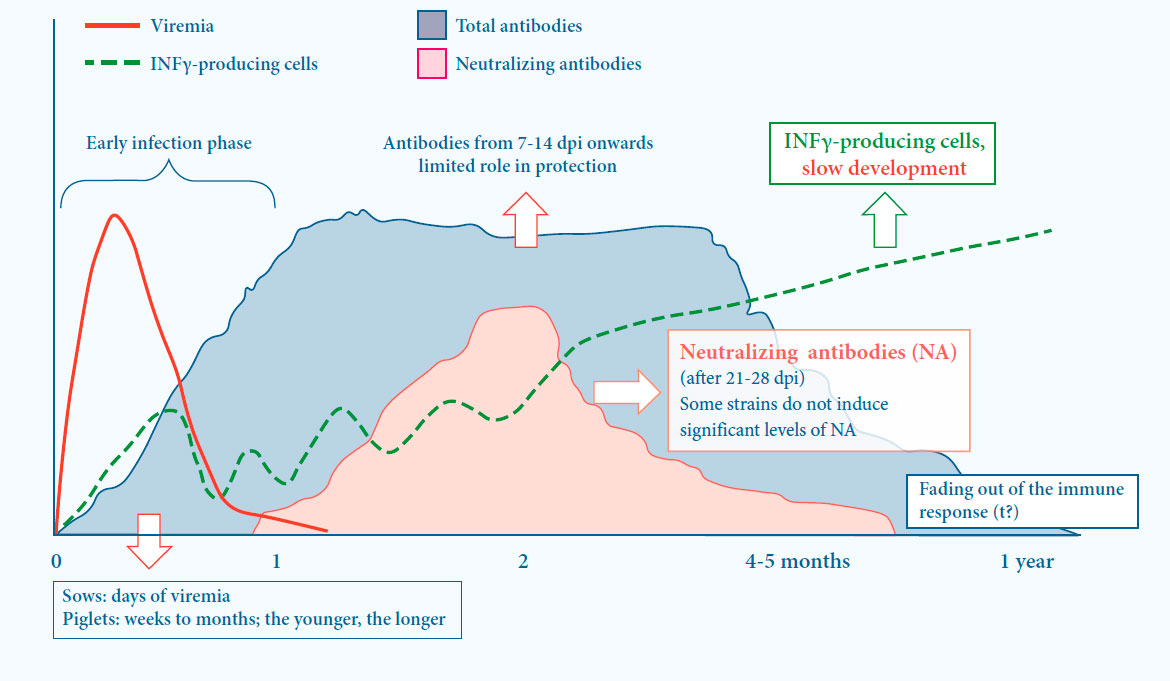
Fig. 3. Viral and immunological dynamics after PRRS infection5
PROGRESSIS® induces CMI and humoral immunity resulting in a broad and eff ective immune response
- PROGRESSIS induces ELISA and seroneutralizing (SN) antibodies6
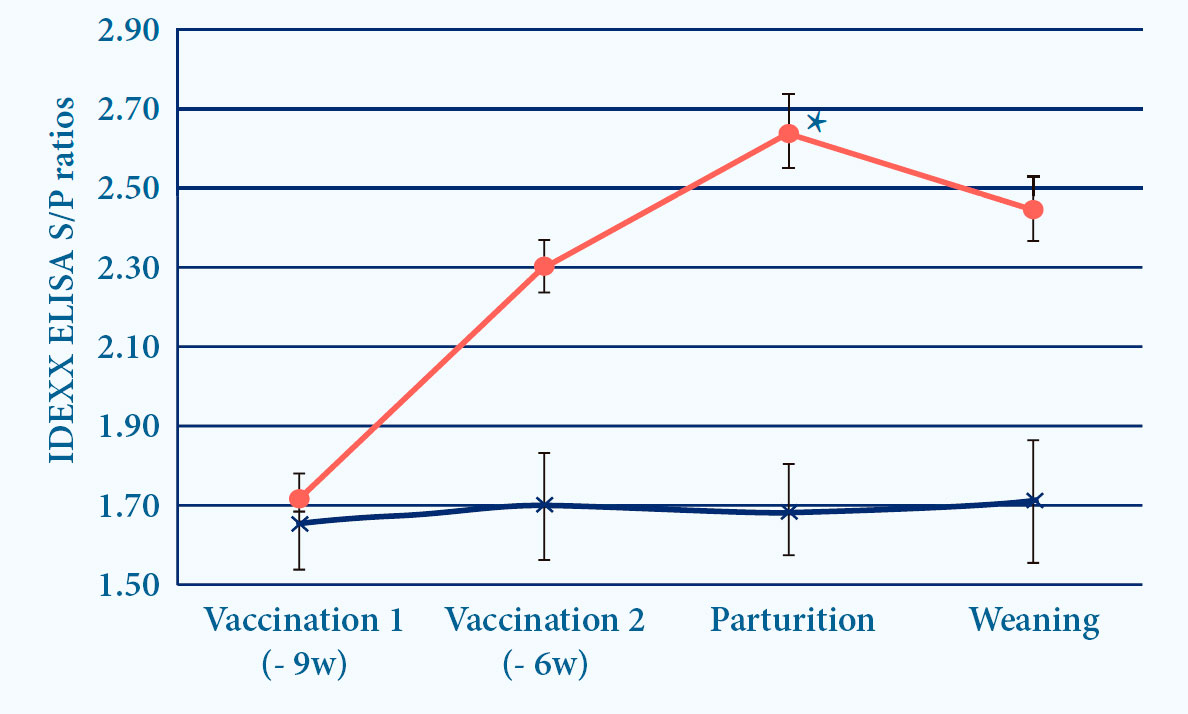
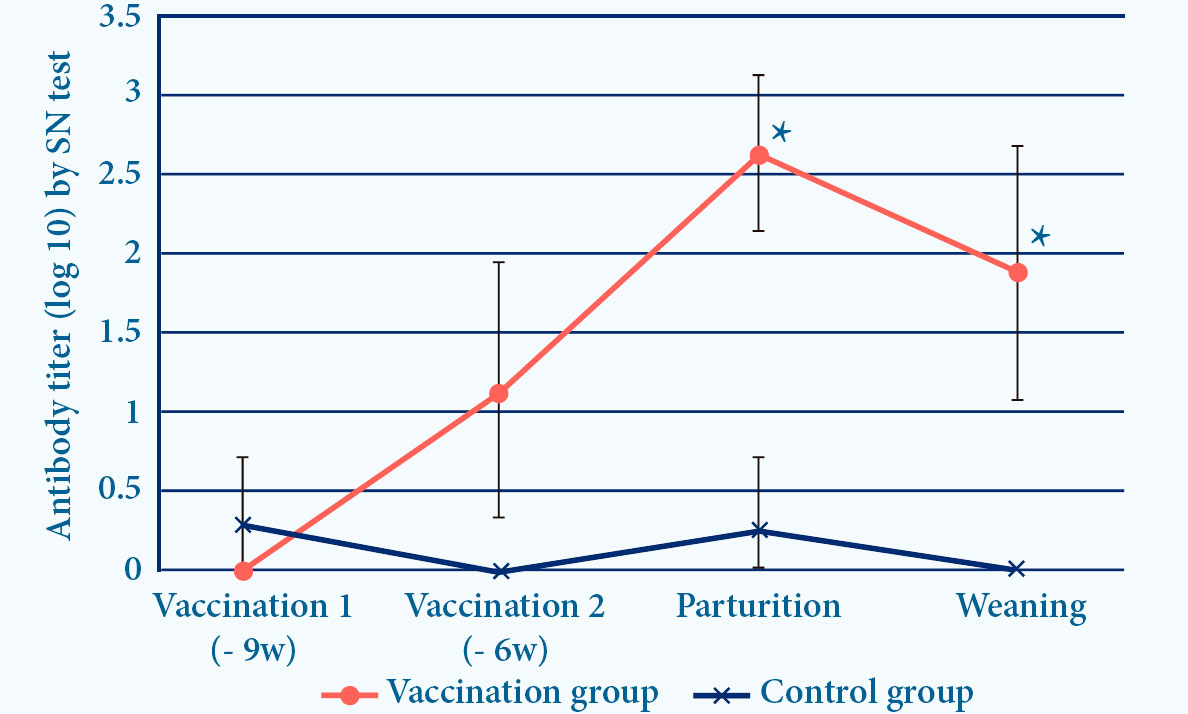
Fig. 4. Progressis induces IDEXX and SN antibodies after vaccination (p<0.05)6.
- PROGRESSIS® induces a strong and early Cell Mediated Immunity (CMI)7
- Induction of Interferon- secreting cells (INF -SC)
- Against a broad range of PRRS fi eld strains, type 1 and type 2
1-6 EU strains (% homology GP5 nucleotides) 7+8 US strains (% homology GP5 nucleotides)
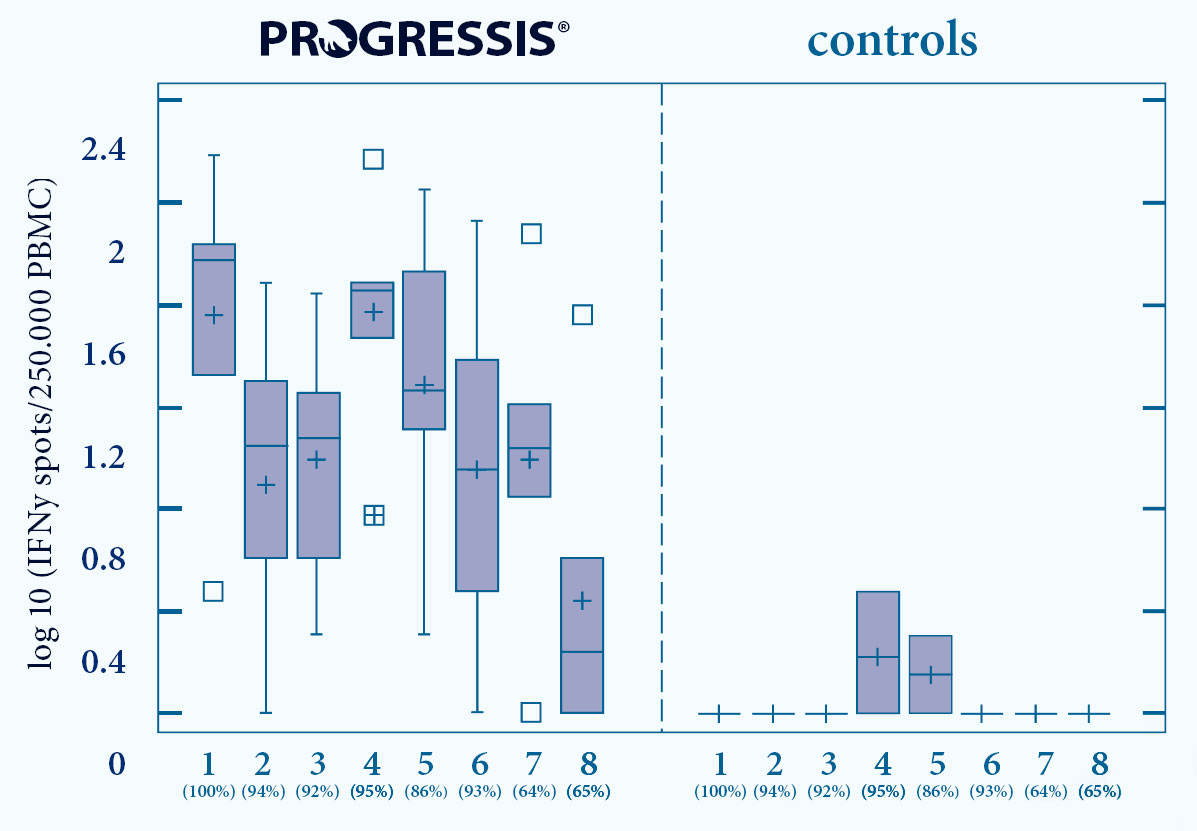
Fig. 5. Progressis vaccinated animals show CMI reaction against a range of diff erent PRRS strains7
Novelty: in vaccination
A new concept: Dual Technology Prime Boost (DTPB)9
- Based on 2 diff erent types of vaccine with the same antigen (eg. MLV and KV vaccine for PRRS)
- Overwhelming experience in diff erent species and infections11
- Induction of broader and stronger immune response9
DTPB immunization strategy results in10:
- Strong cellular immune response
- Higher and more specifi c antibody response compared to homologous immunization
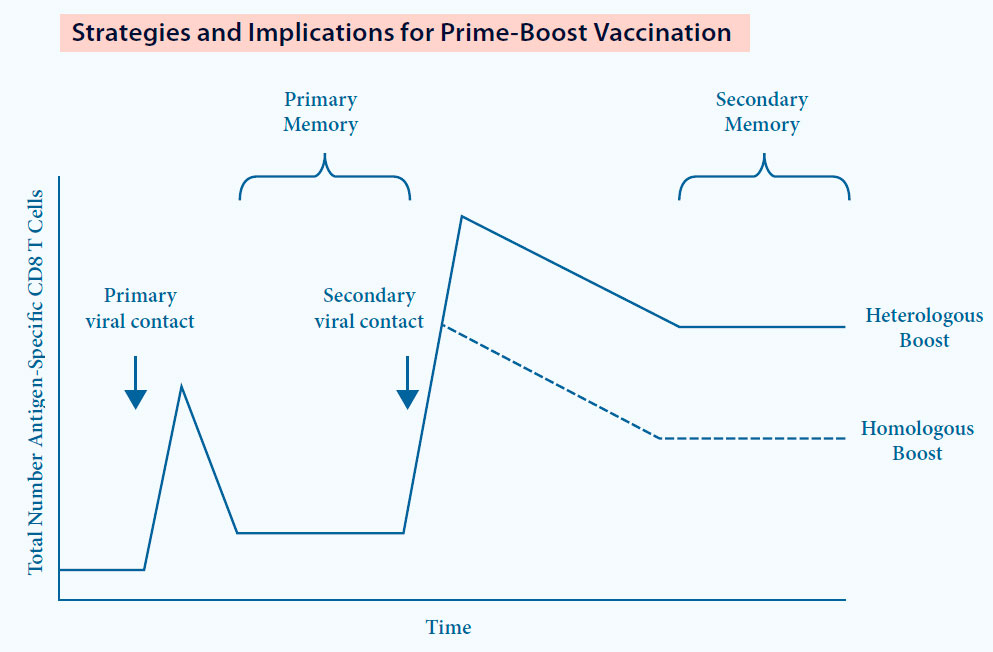
Fig. 6. DTPB can be more immunogenic compared to Homologous Prime Boost.12
In a contaminated environment, PROGRESSIS® boosts the CMI induced by a live PRRS virus8
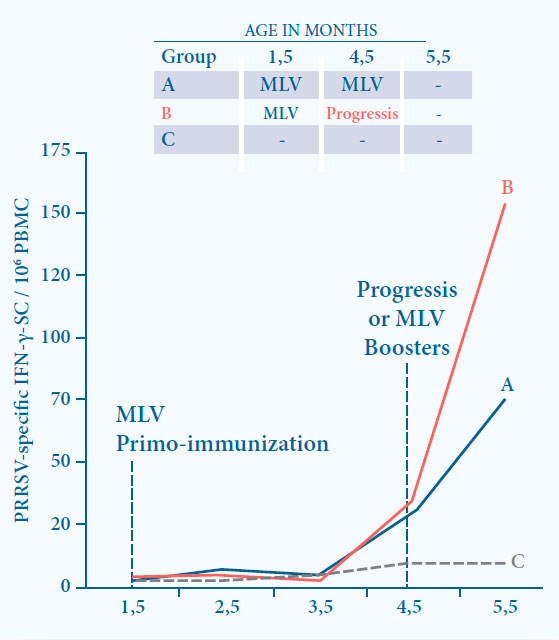
Fig. 7. Boosting the immunity induced by a MLV vaccine by Progressis results
in a significantly higher CMI at 1 month after boost (p<0.05).
DTPB in practice: results from the field
Reproductive performance
In a 2000 sows farm in Belgium, implementing the DTPB concept with Progressis resulted in significant13:
- Increased number of live born piglets
- Reduced piglet mortality before weaning
- Increased number of weaned piglets
These results were confirmed in several studies14,15,16
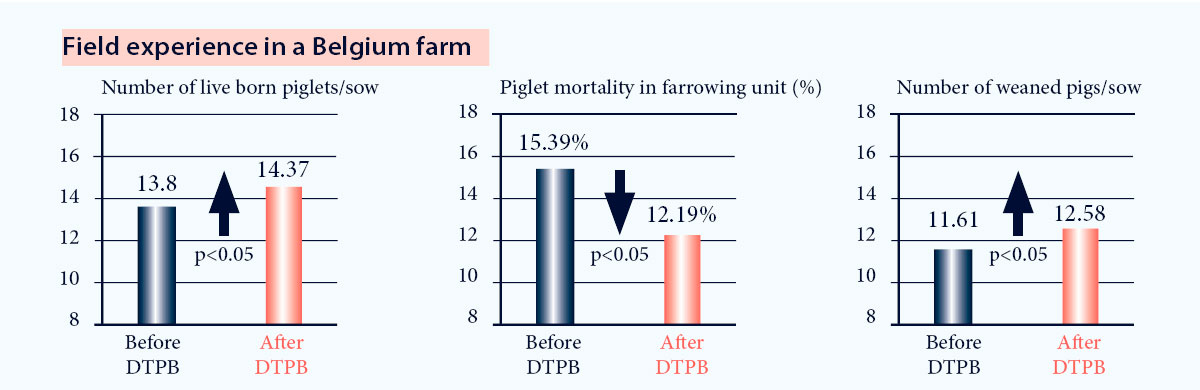
Fig. 8. Improvement of reproductive results after implementation of DTPB program13.
Field monitoring
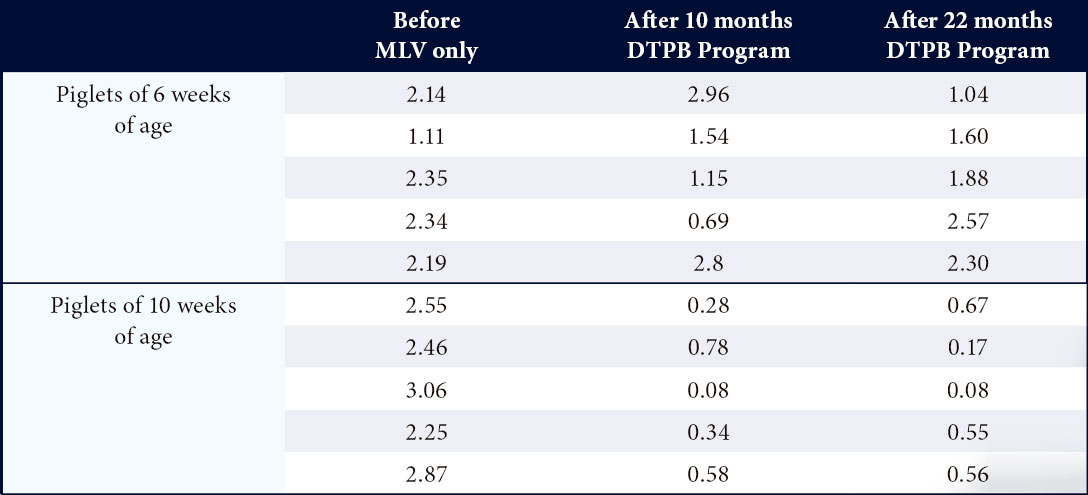
Reducing circulation of PRRS in a 1200 sow farrow to fi nish farm17
- Before the implementation of the DTPB program, PRRS circulation was observed from 3 weeks of age onwards.
- After implementation of the DTPB program, circulation was delayed and the infection was stabilized in sows and piglets.
Table 1. IDEXX PRRS S/P ratios in sera collected from piglets of 6 and 10 weeks of age before and 10 and 22 months after the start of the DTPB program in a 1200 FF farm17.
Boosting the immunity PROGRESSIS® with after priming with a live virus provides a broader and stronger immunity.
References
1. Nieuwenhuis et al., 2012, Economic analysis of outbreaks of porcine reproductive and respiratory syndrome virus in nine sow herds. Vet Rec 170: 225
2. Progressis Notice (SPC) (Country)
3. Reynaud et al., Zootechnical effi cacy of vaccination of gilts and sows with an inactivated PRRS vaccine in a contaminated environment. IPVS 2000: 601
4. Joisel et al., PRRS: vaccination with a killed vaccine - fi eld experience. The Pig Journal 2001, 48: 120-137
5. Lopez and Osorio, 2004, Role of neutralizing antibodies in PRRSV protective immunity, Vet Immunol Immunopathol 102: 155-163
6. a. Kim et al., ELISA antibody response after vaccination with an inactivated EU-typed PRRS vaccine in a Korean farm. APVS 2015: 83
b. Kim et al., Serum neutralization (SN) antibody response in sows and transfer to piglets after sow vaccination with an inactivated EU-typed PRRS vaccine in a Korean Farm. APVS 2015: 84
7. Juillard et al., Ex-vivo stimulation with diff erent PRRSV strains for cellular mediated immunity monitoring of PROGRESSIS vaccinated pigs. ISERPD 2007: 144
8. Diaz et al., 2013, Comparison of diff erent vaccination schedules for sustaining the immune response against porcine reproductive and respiratory syndrome virus. Vet Journal 197: 438-444
9. Meyns et al., The future of PRRSV vaccination: boosting with inactivated vaccine to capitalize on pre-existing immunity, the innovation towards stronger protection. Proc. International PRRSV congress Ghent 2015: 103
10. Delany et al., 2014, Vaccines for the 21st century. EMBO Mol Med, 6 (6): 708–720
11. Lu, 2009, Heterologous prime-boost vaccination. Curr Opin Immunol 21 (3): 346–351
12. Nolz and Harty, 2011, Strategies and implications for prime-boost vaccination to generate memory CD8 T cells. Adv Exp Med Biol 780: 69-83
13. Knockaert et al., Improved reproductive performance after PROGRESSISR vaccination at the end of gestation in a PRRSV infected farm. ESPHM 2015: PO84
14. Willems et al., Stabilization of PRRSV circulation in a nursery and fattening unit following the implementation of mixed PRRSV vaccine program in breeders. ESPHM 2015: PO 74
15. Willems, Benefi cial impact of a PRRSV vaccination program combining a modifi ed live vaccine and PROGRESSISR on virus circulation and technical performance. IPVS 2016: PO-PW1-147
16 Spaans et al., Eff ect of dual technology prime boost vaccination in sows on circulation of PRRSV in post weaning piglets. IPVS 2016: PO-PW1-182
17. Defoort et al., Stabilization of PRRSV circulation in a farm using a vaccination program with PROGRESSISR at the end of gestation. IPVS 2014: 565
Progressis® Emulsion for Injection for Pigs (sows and gilts) contains Inactivated Porcine Reproductive and Respiratory Syndrome (PRRS) virus, P120 strain. The product is indicated for the reduction of the reproductive disorders caused by Porcine Reproductive and Respiratory Syndrome virus (European strain) in a contaminated environment: vaccination reduces the number of early farrowings and the number of stillbirths. In PRRS infected herds, viral infection is heterogeneous and varies over time. In such context, the implementation of a vaccination program is a tool to improve the reproductive parameters and may contribute to the disease control in conjunction with sanitary measures. Amounts to be administered and administration route. One dose of 2 ml is administered by deep intramuscular route, in the neck muscles behind the ear, according to the following vaccination scheme. Primary vaccination:Gilts: 2 injections 3-4 weeks apart, at least 3 weeks before mating. Sows: 2 injections 3-4 weeks apart. Revaccination: One injection at 60-70 days of each gestation, as of the fi rst gestation following the primary vaccination. Withdrawal period: Zero days. Pharmaceutical precautions Use immediately after opening. Store in a refrigerator (2°C – 8°C). Do not freeze. Protect from light.
For more details, see the SPC applicable in your country.
This page contains information on a veterinary biological product sold in several diff erent countries and areas where it may be subject to diff erent regulatory approvals. Ceva gives no guarantee that the details presented are correct with respect to all locations. In addition, the safety and effi cacy data and the withholding periods may be diff erent depending on local regulations. Please consult your veterinarian for further information.